January 23, 2022 is the 100th anniversary of the first successful insulin injection for diabetes in a human. Prior to this, a diagnosis of type 1 diabetes (T1D) meant certain death. On this date, a fourteen-year-old boy named Leonard Thomspon, who was dying from complications of T1D received the first real treatment for the disease. He would live another 13 years. Leonard could be considered the first human in an insulin clinical trial. As a result, millions across the globe can now better manage their diabetes.

This day has special meaning to our family. Our daughter Tilla was just 10 years old when she was diagnosed with T1D in the summer of 2019. Now, like everyone who lives with this disease, her days are a series of decisions, tracking her blood sugar and insulin levels, and measuring her food, particularly carbs. And although we are fortunate to have the best diabetes technology available to us, it can still be immensely stressful at times. It is a 24-7 disease with no days off.
After Tilla’s diagnosis, we started researching available clinical trials that she could be enrolled in, and Tilla bravely rose to the challenge. She enrolled in the PROTECT study, which is testing an investigational medicine called teplizumab – a drug JDRF Canada has supported earlier trials of. It is hoped teplizumab will help people with T1D to continue making more of their own insulin and reduce or even eliminate the need for insulin injections for many months.
PROTECT is a randomized controlled trial involving a placebo group. Despite knowing that Tilla could be placed in the placebo group, we still chose to participate, with the hopes that not only could it change the disease course for Tilla in the short term, but in the future could impact thousands of other people like her who are diagnosed with T1D every year.
Our son Allan is also enrolled in a T1D clinical trial at Sick Kids hospital in Toronto, ON. He has all the antibodies for the disease, which puts him at far greater risk of developing it in the future. Along with his mother, Allan has flown to Toronto twice. He takes one pill per day/ two pills per day on weekends. It is our hope that if he is not receiving the placebo – this treatment might prevent or delay him from developing T1D. And even if it does not, we know that he is still contributing to advancing scientific knowledge of how we might better screen for and prevent diagnoses of T1D.
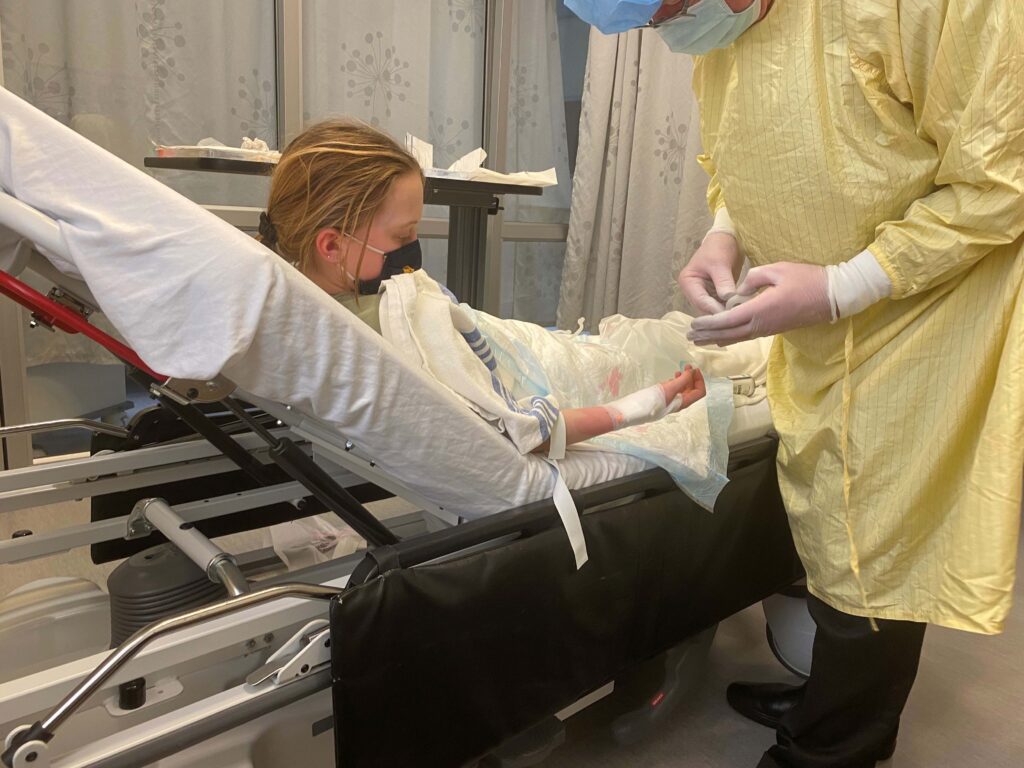
Being involved in clinical research makes us feel as a family that we are directly and personally contributing to helping find a cure for this devastating disease. And we believe this is an important message. Participation in clinical trials is the only way we can get treatments to market and to everyone who can benefit from them. We consider anyone enrolled in clinical trials heroes.
What many people do not know is that the first insulin injection took place on January 11, 1922, but Leonard Thompson had an allergic reaction to it. And that when that first injection did not work, he took another step forward and tried again. From the bravery of one teenage boy, came millions of people whose lives have been saved by insulin. And every year across the globe, people living with T1D celebrate their ‘insulin anniversary’ while waiting for the next breakthrough in diabetes.
Every person who participates in a T1D clinical trial study helps us get closer to a cure. We recognize their courage and are so grateful for their help in accelerating research that will one day mean a world free from diabetes.
We are grateful to JDRF Canada, whose primary goal is funding the most promising research that will move us beyond insulin and toward a cure for T1D. Their investment in this research means that we can participate in these important clinical trials.
Henrik Karlsson, Anna, Tilla and Allan Karlberg, on behalf of JDRF Canada
Vancouver, BC




