JDRF’s #AccessForAll advocacy campaign aims to make type 1 diabetes (T1D) technology affordable and accessible for all Canadians living with the disease. With support from the T1D community, JDRF seeks to increase public and private coverage technologies such as continuous glucose monitors (CGM) and flash glucose monitors (Flash GM). The goal is to reduce the out-of-pocket costs for these technologies and improve access for Canadians with T1D.
To support our advocacy for expanded public coverage, JDRF commissioned a study to examine glucose monitoring systems (flash glucose monitoring, continuous glucose monitoring and self-monitoring of blood glucose) and their population level impact on diabetes related complications, mortality, and cost effectiveness of each of techniques in adults with type 1 diabetes (T1D) in Canada.
An estimated 300,000 people in Canada live with T1D. Living with T1D means managing the disease through frequent checking of blood glucose levels, regular infusions of insulin, either by injection or through an insulin pump, and carefully adjusting each dose to balance against carbohydrate consumption and activity levels.
For years, the only way to measure blood glucose was through frequent and often painful finger-prick tests, and insulin needed to be administered through multiple daily injections. More recently, however, diabetes devices like advanced glucose monitors, like CGMs and Flash GM along with insulin pumps have offered more options for managing diabetes and more accurate readings.
Recently, JDRF commissioned a study to examine the population level impact on diabetes related complications, mortality and cost effectiveness of each of these three glucose monitoring techniques in adults with T1D in Canada. The study used a Markov cost-effectiveness model (a probability-based model that models the probability or chance of transitioning between states and their associated costs) and Canadian epidemiologic and economic data for adults aged 18-64 years with T1D.
The study found the following:
Self-Monitoring of Blood Glucose (SMBG) is an approach whereby an individual pricks their finger, measuring their blood glucose manually using a glucose meter.
Average annual per person costs: $2,019
After 20 years, if all 180,000 Canadians aged 18-64 living with T1D use the traditional finger-prick method, an estimated 11,200 people would remain free of complications and there would be an estimated 89,400 deaths, at a total cost of $12.2 Billion.
A continuous glucose monitor (CGM) works through a tiny sensor inserted under the skin. The sensor measures the glucose level and provides a continuous reading.
Average annual per person costs: $3,930
Universal coverage of CGM would increase the number of people living free of complications by an estimated 7,400 and decrease mortality by an estimated 11,500.
A Flash glucose monitor (Flash GM) measures the glucose level through sensors and provides a blood glucose reading when the user scans the device over the sensor.
Average annual per person costs: $2,540
Universal coverage of Flash GM would allow an estimated 3,400 more people to live without chronic complications of T1D and prevent approximately 4,600 deaths.
Universal use of CGM and/or Flash GM in Canada for the T1D population would reduce complications and death at a cost-effectiveness threshold of $50,000/ Quality adjusted life-years (QALY). QALY is a generic measure of disease burden, including both the quality and the quantity of life lived. It is commonly used in health economic evaluations to measure the cost-effectiveness of medical interventions and is the standard measure of quality of life in cost-effectiveness models. A value of 1 corresponds to one person living one year in perfect health; and a value of 0 is assigned when a person has died.
Quality Adjusted Life-Years (QALY):

Use of CGM and/or Flash GM over SMBG for the T1D population:
- Leads to fewer complications such as:
- Reduces long-term costs associated with hospitalization
- Leads to improved mortality rates
- Are at a much lower cost-effectiveness threshold than the acceptable threshold of $50,000/QALY ($35,017 QALY and $17,488 QALY respectively)
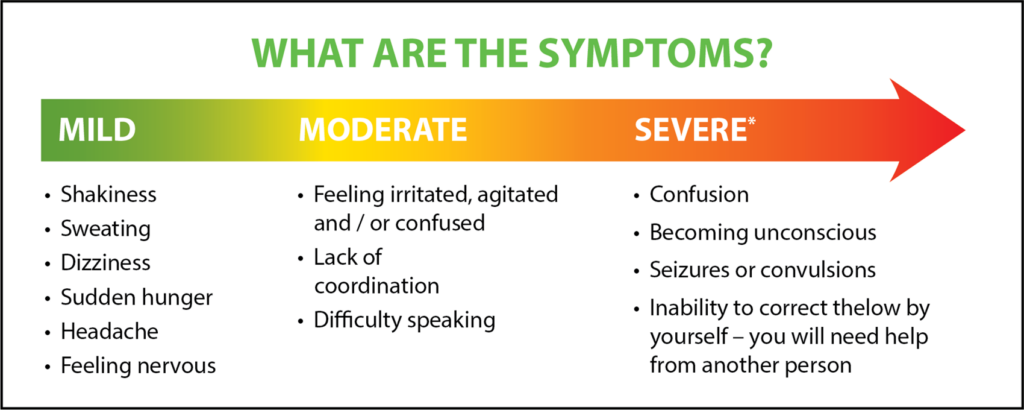
The study demonstrates that universal adoption of CGM and Flash devices would be anticipated to lead to a significant reduction in risk of developing severe hypoglycemia (a severe event where a person often loses consciousness due to low blood glucose), diabetic ketoacidosis (a severe event where a person has high levels of blood glucose and ketones in their blood. These often require hospitalization) and micro- or macrovascular complications (chronic complications that affect the smaller blood vessels such as retinopathy, nephropathy, and neuropathy. They can also lead to other severe complications like kidney disease, blindness, etc.) Macrovascular complications affect more major blood vessels which includes heart attack, coronary artery disease, etc.
Therefore, funding these devices is more cost-effective compared to traditional finger-prick methods, and while the cost of funding CGM and Flash GMs are higher in the near-term, they generate significant long-term cost savings due to lower costs of complications, potential hospitalization, and additional medical interventions.
Moreover, access to these devices brings users greater peace of mind and provides more accurate blood glucose measurements. T1D self-management is facilitated by these technologies through real-time readings that help to improve overall blood glucose (HbA1C) and time in target range (TIR). Better self-management and glucose control also help to relieve some of the anxiety that surrounds the disease. It allows people with T1D to better plan exercise, meals, and rest and greatly improves overall quality of life.
CGM and Flash GM devices are extremely cost-effective compared to traditional finger-prick methods. To learn more about Access for All and how to advocate in your community, please visit https://jdrf.ca/advocacy/access-for-all/