The pace of type 1 diabetes (T1D) research is moving faster than ever before.
In November 2021 to mark November’s National Diabetes Awareness Month, JDRF announced a new investment of $7 million to support four Canadian research teams as part of the JDRF-CIHR Partnership to Defeat Diabetes, which will help to accelerate development of stem cell-based therapies for T1D as well as improve pediatric diabetes research and quality improvement across Canada, and our understanding of variation in human insulin production.
This is part of the results of the Team Grants in Diabetes Mechanisms and Translational Solutions competition, an investment of $20M in 10 research projects. Diabetes Canada, Kidney Foundation of Canada, and the FRQS have also received funding as part of this competition, as all work together to improve health outcomes for Canadians.
JDRF is pleased to share the summaries of all four research grants:

Designing stem cell-derived islets for diabetes therapy
Dr. Timothy Kieffer (University of British Columbia) Nika Shakiba, (University of British Columbia), Dr. Elizabeth Rideout, (University of British Columbia; CIHR Sex and Gender Science), Dr. Corinne Hoesli, (McGill University), Dr. Christopher Moraes (McGill University)
People with type 1 diabetes lack the islet cells that release the hormone insulin. Scientists at the University of Alberta made breakthrough improvements in transplanting clusters of insulin-producing islet cells. The procedure is quick, and many transplant recipients can reduce or even eliminate insulin injections. Unfortunately, the only current source of islets for transplant is recently deceased donors and only a tiny fraction of those in need can receive the procedure.
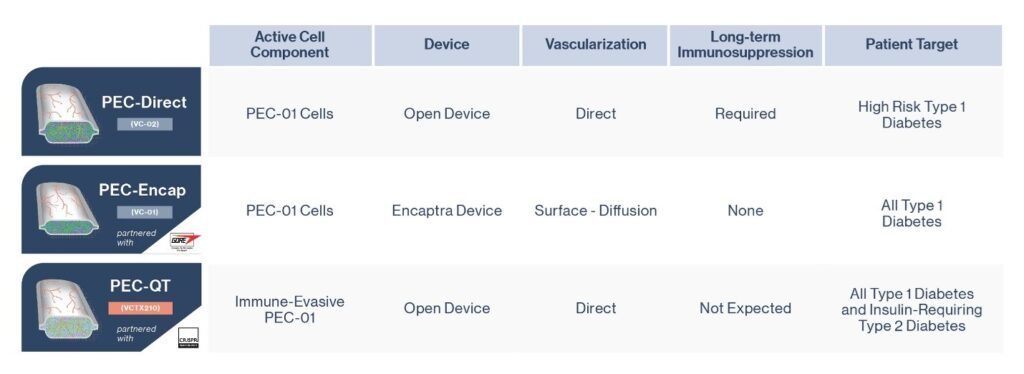
Over the past several years, there have been remarkable breakthroughs in unravelling the process by which islet cells develop naturally in the body. As a result, it is now possible to replicate many steps of this process in the laboratory with cultured stem cells, culminating in insulin-producing cells. Kieffer and his team are aiming to significantly improve upon the manufacturing of the islet cells to obtain more robust insulin delivery, with a focus on generating an optimized process to mass-produce stem cell-derived islet cells that will form the basis for new clinical trials in patients with type 1 diabetes.

A first-in-human trial of autologous induced pluripotent stem cells (ipsc)-derived islets: Developing a personalized diabetes therapy
Dr. James Shapiro, (University of Alberta), Dr. Timothy Kieffer, (University of British Columbia),
Dr. Gregory Korbutt, (University of Alberta), Dr. Patrick MacDonald, (University of Alberta), Dr. Andrew Pepper, (University of Alberta), Dr. Blaire Anderson, (University of Alberta), Dr. Anna Lam, (University of Alberta), Dr. Peter Senior, (University of Alberta), Dr. Khaled Dajani, (University of Alberta)
In type 1 Diabetes (T1D, ~10%), the B-cells are destroyed by one’s own immune system. In type 2 Diabetes (T2D, ~90%), the body becomes more resistant to insulin, increasing the demand and eventually leading to B-cell damage. Shapiro and his team will develop a stem cell-based therapy to replace or supplement damaged B-cells in people with all types of diabetes.
They propose to manufacture new B-like cells from patients’ own blood cells so that they will be accepted by the immune system and no/minimal anti-rejection drugs are needed. In this project, they will conduct a first-in-human trial to implant these cells under the patient’s skin and evaluate their safety and preliminary efficacy.
Being able to transplant an unlimited supply of self-derived islet cells without immunosuppressants is a novel approach to treat all forms of diabetes.
A deep phenotyping network for understanding human islet variation in health and diabetes
Dr. Patrick MacDonald, Nominated Principal Investigator: Canada Research Chair; University of Alberta, along with his team: Dr. James D. Johnson, (University of British Columbia) Dr. Jennifer Bruin, (Carleton University) and Dr. Jianguo (Jeff) Xia, (McGill University).
Insulin is the primary hormone responsible for controlling blood sugar levels. It is produced by the pancreatic islets of Langerhans, rises after a meal to promote energy storage, and falls during fasting to allow energy mobilization. The levels of insulin in the blood vary tremendously amongst people. Nutrition, age, sex, genetics, and environmental exposures are all important factors likely to impact insulin levels. However, the underlying mechanisms by which these factors affect islet insulin production at the cellular level are not clear.
This team seeks to understand the variability in human islet function in relation to genetic and environmental impacts on diabetes risk and to identify mechanisms of islet dysfunction in diabetes. To do this they will take advantage of extensive data on the molecular, cellular, and physiological function of islets from human organ donors. They will also produce tools and resources so that other researchers can explore this data to answer their own questions about islet dysfunction in diabetes.

Building CAPACIty for pediatric diabetes research and quality improvement across Canada
Dr. Shazhan Amed, Nominated Principal Investigator: B.C Children’s Hospital, along with her team:
Dr. Meranda Nakhla, (Montreal Children’s Hospital; McGill University), Dr. Julia von Oettingen, (Montreal Children’s Hospital; McGill University) and Dr. Ian Zenlea, (Trillium Health Partners; University of Toronto).
Although there have been many advances in diabetes care since insulin was discovered 100 years ago, youth with diabetes continue to have a higher risk of other health problems, a lower quality of life, and a shorter life span than their peers without diabetes. This health gap is likely in part due to suboptimal access to and delivery of their diabetes care, which is worse in disadvantaged populations across Canada. This project will develop strategies to address these gaps.
The CAnadian PediAtric diabetes ConsortIum (CAPACIty) is a network of 15 childhood diabetes centers from across Canada. They are partnering with patients/families and health care professionals to jointly design and develop a Canada-wide childhood diabetes registry and research platform. The registry will enable them to improve diabetes care and health outcomes for Canadian youth through comparison of diabetes care quality and outcomes between Canadian diabetes centers, quality improvement initiatives, patient-informed research initiatives across Canada, and successful advocacy work.
They anticipate that the CAPACIty registry will not only lead to better health outcomes but also serve as a powerful tool for governments and decision-makers to implement policy decisions that are driven by our data. Lastly, the patient advisory board will ensure better representation of youth with diabetes and their parents among provincial and national associations that advocate for people living with diabetes.
As we celebrate the centenary of the first successful insulin shot in 2022, a groundbreaking achievement that saved millions of lives, we recognize the need to continue investing in research that will move us beyond insulin treatment towards a cure.
These new grants are an important step in that direction.
To read more about all the JDRF-CIHR Partnership to Defeat Diabetes: www.jdrf.ca/research/jdrf-cihr-funded-projects/